Solid-state Zinc-air Battery
Solid-state Zinc-air Battery (ssZAB)
Why zinc-based batteries?

- Zinc reserves in North America: 40% of the world supply (0.38% for Lithium)
- Zinc world reserves capacity: 1 billion electric vehicles (10 million for Lithium)

- Zinc reserves in North America: 40% of the world supply (0.38% for Lithium)
- Zinc world reserves capacity: 1 billion electric vehicles (10 million for Lithium)
Science 372, pp890-891 (2021)
Mechanism of ssZAB
- ZABs charge and discharge the electrochemical energy by evolving and storing oxygen
- At charging, oxygen is reduced to OH ions at the cathode, and the OH ions transport to the anode to form ZnO

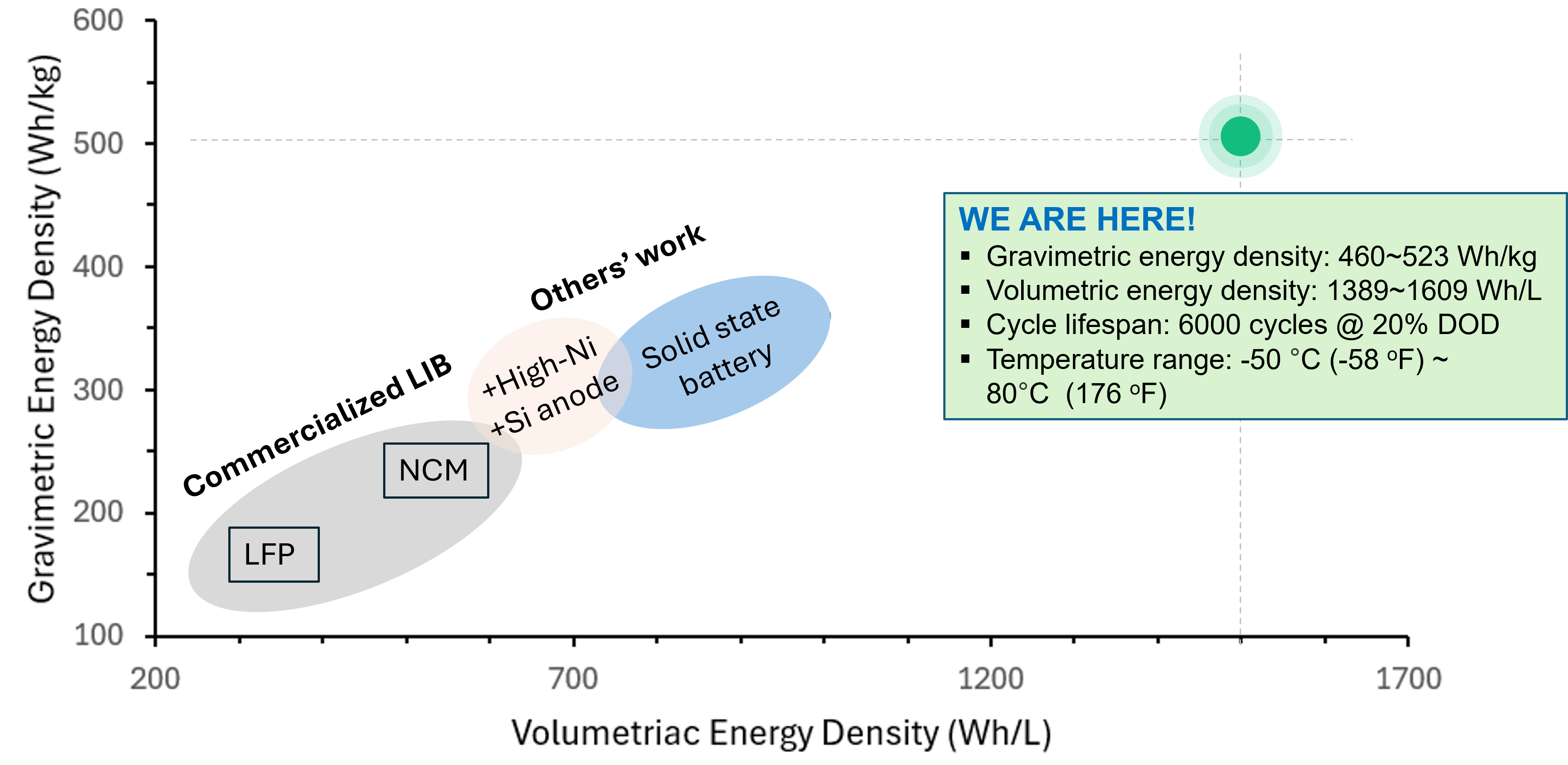
Pouch Cell and Layer Structure
- ssZAB pouch cell with 1 Ah, 460 Wh/kg (left)
- Symmetric cell design (right): bifunctional oxygen catalyst cathode (copper phosphosulfide, CPS), solid membrane electrolyte (chitosan bacterial cellulose, CBC), and surface-treated zinc anode
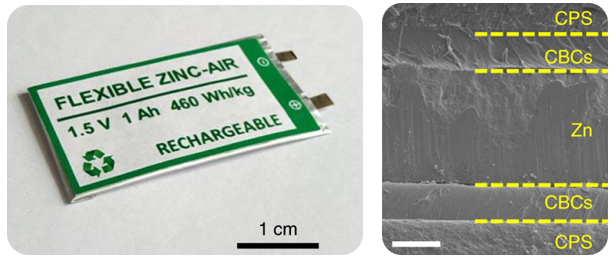
Our Battery Performance as Compared to Other Battery Systems
Lincdyne’s ssZAB achieves twice as high energy density in the same mass and two to three times higher in the same volume as lithium ion batteries